We Provide Cooling Tower Solution
English
Please Choose Your Language
Views: 0 Author: Site Editor Publish Time: 2025-12-18 Origin: Site








Cooling towers play a crucial role in industrial and power generation systems by rejecting waste heat through evaporation. However, maintaining proper water chemistry—especially **pH stability—is essential for efficient operation and long service life. A rising pH in a water cooling tower can disrupt system balance, cause scaling, corrosion issues, and increase operating costs. This article explores the causes, impacts, monitoring, and mitigation of pH increases in cooling towers, aligned with key parameters like cooling tower water supply, cooling tower water flow rate, cooling tower water management, cooling tower water requirements, cooling tower water usage, and water cooling tower system design.

pH is a measure of the hydrogen ion concentration in water, indicating acidity (pH < 7), neutrality (pH = 7), or alkalinity (pH > 7). In cooling tower water management, the ideal pH range is typically 7.0–9.0 depending on system design and treatment strategy.
In a water cooling tower system, water quality directly affects:
Heat transfer efficiency
Scale formation
Corrosion rates
Biological growth
Chemical treatment effectiveness
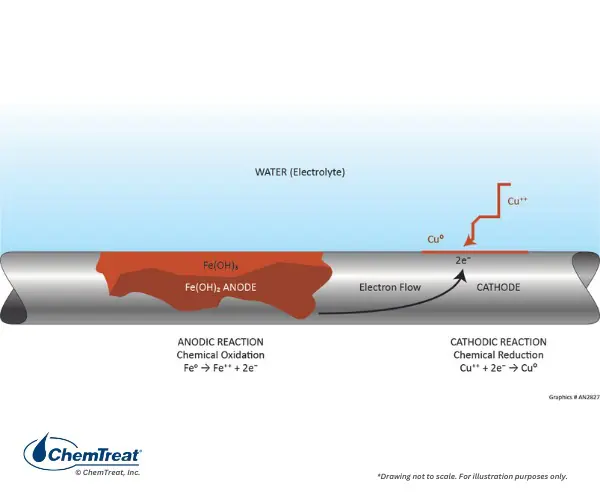
An increase in pH can reduce corrosion but promote scale and fouling, which harms heat transfer and system reliability. Therefore, understanding why pH increases is essential for effective cooling tower water management.
Cooling towers operate by evaporating a portion of the circulating water to remove heat. As water evaporates, dissolved minerals and chemical species become more concentrated in the recirculating water—this can lead to a rise in pH if alkaline species concentrate faster than acidic species are replenished.
Water treatment chemicals are added to control corrosion, scale, and biological growth. Over-dosing alkaline corrosion inhibitors, sequestrants, or buffering agents (e.g., sodium hydroxide or sodium carbonate) can raise pH beyond desired cooling tower water requirements.
The cooling tower water supply (makeup water) may have high alkalinity or high bicarbonate content. As makeup water is introduced to replace evaporation and blowdown losses, it can increase the overall alkalinity and pH of the system.
Blowdown removes concentrated water and dissolved solids to control conductivity and scaling. Reducing blowdown to conserve cooling tower water usage (lower fresh water demand) without adjusting treatment can lead to elevated pH due to accumulation of alkaline species.
When pH rises, scale-forming compounds such as calcium carbonate (CaCO₃) and magnesium hydroxide (Mg(OH)₂) become less soluble and precipitate on heat transfer surfaces.
Example: Scaling Table
| Parameter | Effect at High pH |
|---|---|
| Calcium Carbonate Solubility | Decreases → Scale |
| Heat Transfer Efficiency | Decreases |
| Pressure Drop | Increases |
| Energy Usage | Increases |
| Maintenance Frequency | Increases |
Scale acts as an insulator, reducing cooling efficiency and increasing energy consumption.
Higher pH can reduce general corrosion of carbon steel but may increase localized corrosion (e.g., under deposits). Often, corrosion inhibitors are adjusted based on pH, so an unplanned increase can leave metals unprotected or promote unexpected corrosion patterns.
While many microbes prefer neutral to slightly acidic environments, some organisms thrive at higher pH. Elevated pH may also reduce the effectiveness of biocides, complicating cooling tower water management.

Frequent testing of:
pH
Conductivity
Alkalinity
Temperature
Turbidity
is essential in any water cooling tower system design to maintain cooling tower water requirements and prevent undesirable deviations.
Monitoring Tips:
Measure pH at multiple points (basin, return line)
Use calibrated, reliable probes
Record results consistently to identify trends
If pH increases:
Reduce alkaline dosages
Introduce controlled acid addition (e.g., dilute sulfuric acid) as corrective measure
Use buffering agents that stabilize pH within a target range
Maintaining proper blowdown prevents accumulation of alkaline species. Adjusting blowdown frequency and volume based on cooling tower water flow rate and cycles of concentration helps control pH without excessive chemical use.
Makeup water (cooling tower water supply) should be of consistent and predictable quality. When possible, pre-treat makeup water (softening or dealkalization) to reduce influx of high alkalinity.
Automated controllers with real-time pH feedback can adjust chemical feed rates and blowdown valves dynamically, maintaining pH within desired limits and enhancing efficiency.
Materials resistant to changes in pH and corrosion—such as stainless steels, duplex alloys, or FRP components—can mitigate long-term damage from oscillations in water chemistry.
Proper design of cooling tower water flow rate, basin geometry, and drift eliminators minimizes dead zones and uneven chemistry distribution. Well-designed recirculation ensures that the cooling tower water usage and treatment are effective throughout the entire water cooling tower system.
MachCooling (https://www.machcooling.com/) is a professional water cooling tower system manufacturer offering engineered solutions tailored to precise water chemistry management, including pH control:
Custom designs matching specific cooling tower water flow rate and capacity.
Comprehensive water treatment strategies integrated with tower systems.
Solutions that meet stringent cooling tower water requirements for industrial and commercial applications.
Expertise in cooling tower water management to optimize cooling tower water usage and minimize chemical inefficiencies.
Systems that accommodate variations in cooling tower water supply quality with targeted treatment modules.
Visit MachCooling at https://www.machcooling.com/ to explore advanced cooling tower options that deliver stable water performance and long-term reliability.
Frequent water chemistry testing helps detect pH increase early and prevent downstream issues.
Maintain chemicals within designed parameters and adjust based on real-time water data.
Control cycles of concentration to avoid excessive accumulation of alkaline species.
Invest in robust designs and materials compatible with pH fluctuations and water chemistry dynamics.
An increase in pH within a cooling tower can arise from simple evaporation effects to complex chemical imbalances, makeup water quality, and system management decisions. Understanding these causes and implementing proactive cooling tower water management and monitoring protocols ensures stable operation, efficient heat rejection, and longer equipment life.
The right water cooling tower system, designed with suitable cooling tower water requirements and supported by expert partners like MachCooling, helps industries avoid pH-related issues—leading to optimized performance and reduced maintenance.
Explore reliable cooling tower solutions at https://www.machcooling.com/.