We Provide Cooling Tower Solution
English
Please Choose Your Language
Views: 0 Author: Site Editor Publish Time: 2025-12-12 Origin: Site








Effective cooling tower water treatment is essential for maintaining performance, extending equipment life, reducing corrosion, preventing scale and biological growth, and minimizing maintenance costs. Cooling tower water treatment chemicals play a central role in these objectives—especially in systems such as a water cooling tower, water cooling tower system, water cooled tower, and closed loop cooling tower. This article explains the key chemical groups used, how they work, and best practices for their application. It also highlights how quality solutions from manufacturers like Mach Cooling (https://www.machcooling.com/) support optimized water treatment performance.
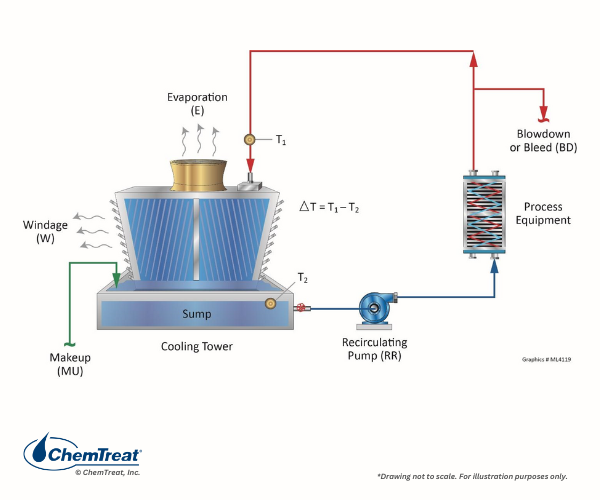
Cooling towers reject heat through water evaporation. As water recirculates, dissolved minerals, corrosion byproducts, and microorganisms accumulate. Without proper treatment, this leads to:
Scale formation (mineral deposits on heat transfer surfaces)
Corrosion (metal degradation in piping and equipment)
Microbiological growth (biofilm and Legionella risk)
Reduced heat transfer efficiency
A well-designed cooling tower water treatment program uses a combination of cooling tower water treatment chemicals tailored to water quality, operating conditions, and tower type (open vs. closed loop).
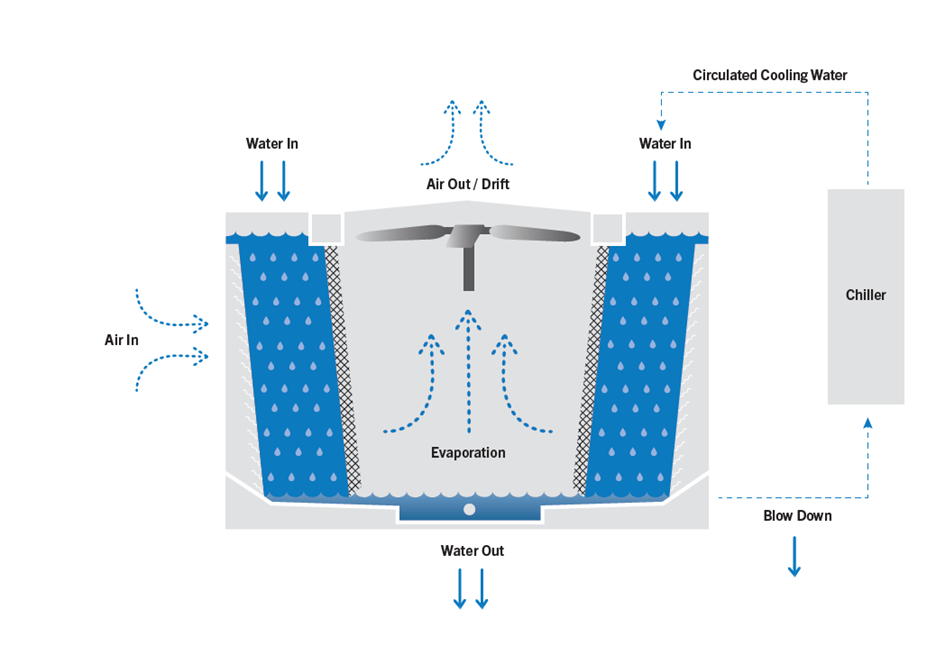

When hard water evaporates, dissolved minerals (like calcium and magnesium) precipitate and form scale, which impedes heat transfer.
Metal components—including steel, copper, and aluminum—can corrode due to oxygen, conductivity, and aggressive ions in the water. Corrosion inhibitors protect these surfaces.
Warm, recirculating water in cooling towers is an ideal environment for bacteria and algae. Biocides reduce these risks and help prevent biofilms that hinder heat transfer and can harbor pathogens like Legionella.
“Cycles of concentration” refer to the ratio of dissolved solids in circulating water relative to makeup water. Good chemical treatment allows higher cycles (less blowdown), saving water while controlling scale and corrosion.

Scale inhibitors prevent mineral crystals from forming and depositing on heat exchange surfaces. Common chemicals include:
Phosphonates
Polymeric dispersants (e.g., polyacrylates)
Threshold inhibitors
These chemicals bind ions like calcium and magnesium or disrupt crystal growth, reducing scale formation even at higher concentration cycles.
Corrosion inhibitors form a protective film on metal surfaces, reducing oxidation and metal loss. Types include:
Azoles (e.g., benzotriazole for copper alloys)
Nitrites (for ferrous surfaces)
Molybdates
Phosphate-based inhibitors
Corrosion inhibitors are especially important in systems that combine different metals (e.g., copper and steel), which can create galvanic corrosion.
Biocides control bacteria, algae, and slime. They are typically categorized as:
Oxidizing biocides
Non-oxidizing biocides
| Type | Examples | Primary Function |
|---|---|---|
| Oxidizing | Chlorine, chlorine dioxide, bromine | Rapid kill of broad spectrum microbes |
| Non-oxidizing | Glutaraldehyde, isothiazolinones, quats | Long-lasting control of biofilms and resistant organisms |
Biocides are often applied intermittently (shock dosing) or continuously at low levels.
Dispersants keep suspended solids and sludge in suspension so they can be removed by blowdown. Common dispersants include:
Polyacrylic acids
Sulfonated polymers
These chemicals help prevent fouling and maintain efficient heat transfer surfaces.
Maintaining a stable pH (typically 7–8.5) helps optimize the performance of other chemicals and reduces corrosion. Common agents:
Sodium hydroxide (raises pH)
Sulfuric or hydrochloric acid (lowers pH)
Foam can develop due to organics or entrained air. Anti-foaming agents (silicone or organic compounds) reduce foam formation.
Chelants (like EDTA or citrates) bind metal ions, preventing them from participating in scale formation or corrosion reactions.
In open systems like a water cooling tower or water cooled tower, evaporation leads to rapid concentration of dissolved solids. Typical treatment includes:
Scale inhibitors
Corrosion inhibitors
Oxidizing and non-oxidizing biocides
Dispersants
Due to higher evaporation rates and blowdown requirements, these systems often require robust chemical monitoring and control.
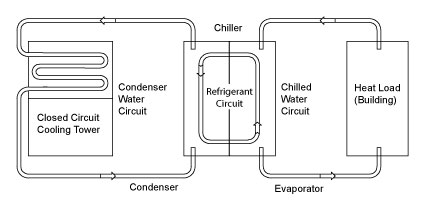
Closed loop cooling towers circulate water inside a heat-exchange coil separated from the air stream. Although direct exposure to contaminants is reduced, treatment is critical to prevent scale on coil surfaces.
Closed-loop treatment focuses on:
Scale inhibitors (to protect heat exchanger surfaces)
Corrosion inhibitors (to prolong tubing life)
pH control agents
Biocides may be used if any open recirculation or makeup water quality issues arise.
Chemicals may be dosed in two main ways:
Batch dosing: Periodic addition of concentrated chemicals.
Continuous dosing: Small, consistent feed using chemical metering pumps.
Continuous dosing provides more stable control and often reduces overall chemical usage.

Water treatment effectiveness is monitored through:
pH measurement
Conductivity / TDS
Oxidation-Reduction Potential (ORP)
Bacteria counts (e.g., ATP testing)
Langelier Saturation Index (LSI) for scale potential
Automated controllers can integrate sensors with chemical feed pumps, improving responsiveness and consistency.
| Chemical Type | Purpose | Common Examples |
|---|---|---|
| Scale Inhibitors | Prevent mineral deposits | Phosphonates, polyacrylates |
| Corrosion Inhibitors | Protect metals | Azoles, nitrites, molybdates |
| Oxidizing Biocides | Broad microbial control | Chlorine, bromine |
| Non-oxidizing Biocides | Biofilm control | Glutaraldehyde, isothiazolinones |
| Dispersants | Prevent particle buildup | Polyacrylic acids |
| pH Adjusters | Maintain stable pH | Acids & bases |
| Anti-Foam Agents | Reduce foam | Silicone org. compounds |
Cause: High hardness or silica.
Solution: Use strong scale inhibitors and dispersants; adjust blowdown to manage cycles of concentration.
Cause: Low pH, high chloride, oxygen ingress.
Solution: Corrosion inhibitors, oxygen scavengers (e.g., sulfites), pH control.
Cause: Warm temperatures, nutrients in water.
Solution: Combined oxidizing and non-oxidizing biocide program; regular monitoring.
Every system—including water cooling tower system and closed loop cooling tower installations—has unique water quality and loading. Treatment should be tailored to makeup water chemistry, heat load, and environmental regulations.
Monitor pH, conductivity, ORP, and microbial indicators frequently. Automated systems improve consistency and reduce human error.
Good treatment integrates with blowdown scheduling, conductivity control, and maintenance routines.
Manufacturers like Mach Cooling (https://www.machcooling.com/) provide more than just equipment—they support comprehensive cooling tower water treatment strategies by offering:
Expert guidance on cooling tower water treatment chemicals selection
Engineering support for integrating treatment systems with cooling towers
Turnkey solutions for water cooled tower and closed loop cooling tower projects
Effective treatment improves heat rejection, reduces operating cost, and extends equipment service life.
Proper use of cooling tower water treatment chemicals is vital for maintaining performance in any cooling system. From scale inhibitors and corrosion control agents to biocides and dispersants, each chemical group addresses specific challenges related to water quality and heat transfer. A well-designed treatment program helps:
Control scale and corrosion
Prevent biological growth
Improve heat transfer and efficiency
Extend the life of the water cooling tower system
Partnering with experienced suppliers like Mach Cooling strengthens operational reliability and ensures your cooling systems run efficiently and sustainably.